What comes out of a car’s exhaust pipe?
Car exhaust is also called vehicle emissions. It contains many substances. Some are more harmful than others.
Your car’s engine probably uses gasoline as fuel. Gasoline is a hydrocarbon. Your car mixes this fuel with air before burning it. This process is called combustion, and it produces many chemical by-products.
Some of these by-products are perfectly safe. For example, air consists of 78% nitrogen gas (N2). Some of this nitrogen reacts with oxygen during combustion. However, most of it gets released as N2 in the engine’s exhaust. Engine exhaust also includes water (H2O). You’ll often see water dripping from exhaust pipes during the winter.
Car engines also emit a lot of harmful substances. Some of these can cause acid precipitation. This is the case with carbon dioxide (CO2), nitrogen oxides (NOx) and sulphur oxides.
Other vehicle emissions can cause health problems like cardiovascular disease and cancer. This is the case with unburnt hydrocarbons, particulates (carbon particles), and volatile organic compounds (VOCs).
Car engines also release carbon monoxide (CO). This poisonous gas can replace oxygen in your bloodstream. If you breathe enough of it, it can even suffocate you!
That all sounds very dangerous, doesn’t it? Fortunately, catalytic converters help make engine emissions less harmful. Here’s how.
What is a catalytic converter?
The catalytic converter was invented around 1950 by Eugène Houdry. He was a French mechanical engineer. He designed the catalytic converter to clean up automobile exhaust.
Widespread use of catalytic converters began around 1975. At that time, governments started trying to reduce air pollution from cars. But back then, a lot of vehicles used leaded gasoline. Lead (Pb) can keep a catalytic converter from functioning properly. That’s because lead can coat the surface that normally reacts with the exhaust gases.
How do catalytic converters work?
On a car, the catalytic converter is attached to the exhaust pipe. A metal casing contains a ceramic honeycomb. The honeycomb is coated with a mix of platinum (Pt), palladium (Pd) and rhodium (Rh). These noble metals are good at resisting oxidation, corrosion and acid. That means they can stand up to bad weather and all the chemicals released by a car engine.
The noble metals in catalytic converters act as catalysts. Catalysts are compounds that can trigger a chemical reaction without being affected themselves. The honeycomb structure inside a catalytic converter maximizes the surface area where reactions can take place.
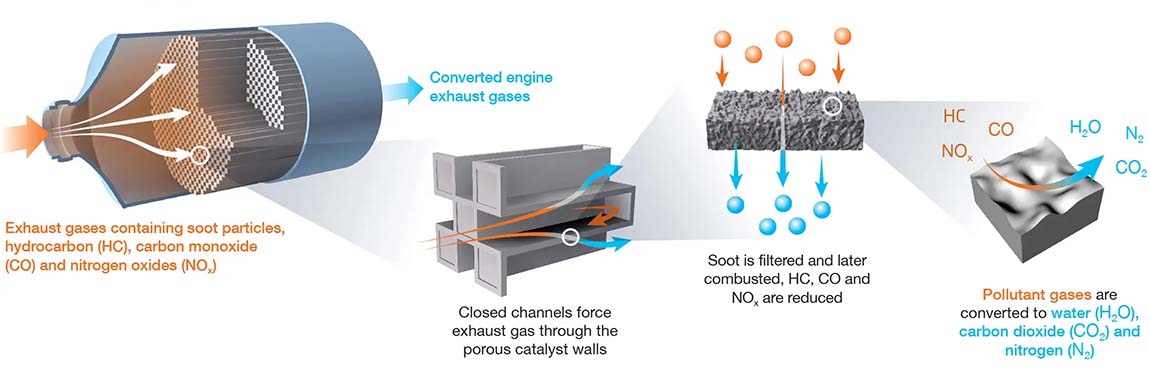
What chemical reactions happen in a catalytic converter?
Catalytic converters use reduction and oxidation (redox) reactions to reduce harmful emissions.
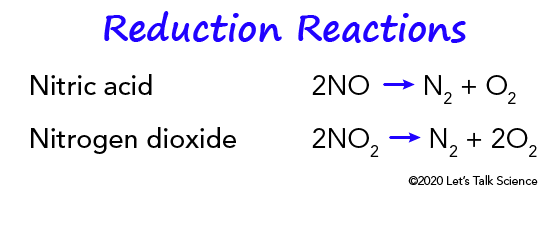
They use a reduction catalyst composed of platinum and rhodium. It helps reduce nitrogen oxides (NOx) by removing nitrogen atoms from nitrogen oxide molecules (NO and NO2). This lets the free oxygen form oxygen gas (O2). Then, the nitrogen atoms attached to the catalyst react with each other. This reaction creates nitrogen gas (N2).
Catalytic converters also use an oxidative catalyst composed of platinum or palladium. It helps reduce hydrocarbons (HC) and carbon monoxide (CO). To start with, carbon monoxide and oxygen combine to form carbon dioxide (CO2). Then, unburnt hydrocarbons and oxygen combine to form carbon dioxide and water.
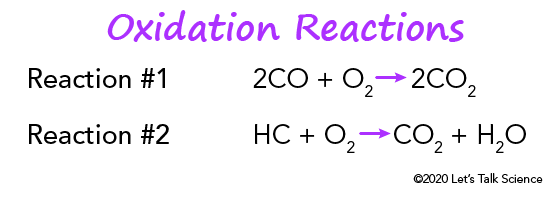
Modern catalytic converters also use oxygen sensors. They’re sometimes called lambda sensors. They control how much extra oxygen gets pumped into the exhaust stream. Maintaining the correct amount of oxygen makes the reduction and oxidation reactions more efficient.
Exhaust Distribution has greatly selection of direct Fit
Catalytic Converter, we are Top rated seller, checkout exhaust distribution
catalytic converter review exhaust
distribution catalytic converter review
The information provided is for educational purpose only,
provided by www.letstalkscience.ca
